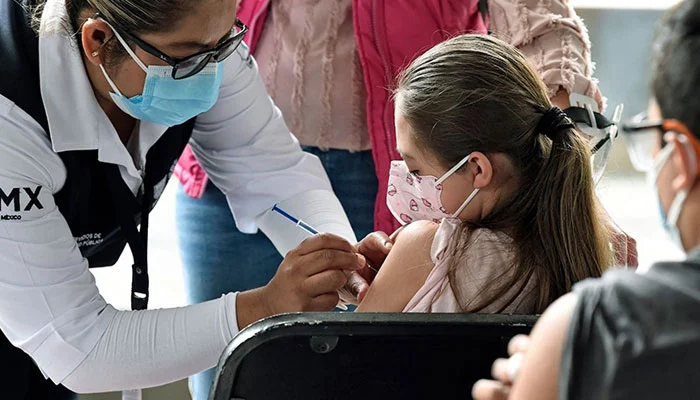
WASHINGTON: A board of specialists met by the US Food and Drug Administration collectively suggested Covid-19 immunizations Wednesday for youngsters under five, the last age bunch anticipating vaccination in many nations.
Formal approvals for Moderna and Pfizer ought to follow soon, with the first shots in quite a while expected right on time one week from now, a little more than eighteen months after the principal Covid immunizations were greenlighted for the older in December 2020.
“This suggestion fills a huge neglected need for a truly overlooked more youthful populace,” said Michael Nelson, a teacher of medication at the University of Virginia, one of the 21 specialists requested to decide in favor of the achievement meeting.
Dissimilar to controllers in different nations, FDA offers livestreams of its interior considerations and its blessing is viewed as the worldwide highest quality level.
Opening the conversation, senior FDA researcher Peter Marks expressed that in spite of studies showing most of kids have now been contaminated with the Covid, the high pace of hospitalizations among babies, babies and small kids during the previous winter’s Omicron wave highlighted a pressing requirement for immunization.
“We are managing an issue where we must be cautious we don’t become numb to the pediatric passings as a result of the staggering number of more established passings,” he said.
“Each life is significant and antibody preventable passings are something we might want to attempt to take care of.”
The United States has recorded 480 Covid passings in the 0-4 age bunch in the pandemic – – far higher than even a terrible influenza season, Marks said.
As of May 2022, there have been 45,000 hospitalizations in that gathering, almost a fourth required serious consideration.
In front of the gathering, the FDA posted its autonomous examinations of the drug organizations’ immunizations, considering both protected and viable.
The two antibodies depend on courier RNA, which conveys hereditary code for the Covid spike protein to human cells that then, at that point, develop it on their surface, preparing the invulnerable framework to be prepared. The innovation is currently viewed as the main Covid immunization stage.
Pfizer looked for approval for three dosages at three micrograms given to kids matured a half year through four years, while Moderna requested the FDA to approve its immunization as two portions of a higher 25 micrograms for a very long time a half year through five years.
The two antibodies were tried in preliminaries of thousands of kids. They were found to cause comparative degrees of gentle aftereffects as in more established age gatherings and set off comparable degrees of antibodies.
– Two dosages, or three? –
Adequacy against contamination was higher for Pfizer, with the organization putting it at 80%, contrasted with Moderna’s assessments of 51% for youngsters matured a half year to two years of age and 37 percent for those matured two to five years.
Yet, the Pfizer figure depends on not very many cases and is accordingly viewed as starter. It likewise takes three dosages to accomplish its assurance, with the third shot given two months after the second, which is given three weeks after the first.
Moderna’s immunization ought to give solid security against serious infection following two dosages, given a month separated, and the organization is considering adding a supporter that would raise viability levels against gentle illness.
In any case, Moderna’s choice to go with a higher portion is related with more elevated levels of fevers in response to the immunization contrasted with Pfizer.
There are approximately 20 million US youngsters matured four years and under.
Despite the fact that weight, neurological issues and asthma are related with expanded hazard of extreme illness among small kids, foreseeing serious outcomes is difficult.
Truth be told, 64% of hospitalizations in those under five happened in patients without comorbidities.
Kids can likewise proceed to contract multisystem fiery disorder in youngsters (MIS-C), an uncommon however serious post-viral condition. Exactly three to six percent can encounter long Covid side effects for over 12 weeks.
The FDA is supposed to before long follow up on the board’s proposal, and the matter will go to the Centers for Disease Control and Prevention for a last say.
White House authorities last week said the rollout of 10 million shots at drug stores and specialists’ workplaces could start when June 21.